Immunotherapy of Mastopathy and Breast Cancer
 What are Mastopathy and Breast Cancer?
What are Mastopathy and Breast Cancer?
Mastopathy
According to the definition published by WHO (1984), mastopathy is the fibrocystic disease (FCD) of breast, characterized by a disbalance between epithelial and connective tissues growths with high proliferative and regressive changes of the breast tissue.
During the histological research of breast of women at the age of 20-40, who die with different reasons, the dishormonal changes are found out in 60-80% of cases. In 30-40 % of cases, mastopathy (dishormonal hyperplasias of mammas) is located during palpation of breast.
FCD is a benign disease, however in many case this pathology can be a transient stage in malignant tumor development process. Since the benign conditions and breast cancers have many similar etiologic factors and pathologic processes, they have common risk factors. In fact, no single risk factor for developing the pathologies are specified- since they are multifactor diseases related with genetic factors as well as external factors.
The hypothalamus-hypophysis system plays an important role in the development of dishormonal hyperplasias of breast. The impairments in the neuro-hormonal controls of reproductive cycles lead to activation of proliferative process in the hormone-dependant organs, including the tissues of breast, which are the target areas for the steroid hormones of ovary, prolactin, placental hormones and implicitly other endocrine hormones. Estrogen has major impacts on the proliferation of epithelial acynus, lobules and interlobular ducts; where as the androgens has more impacts on the fibrotic process [1, 2].
Many women do not pay much attention to mastopathy as it does not seem to be a very serious disease. In fact, the dishormonal hyperplasia of mammas render considerable impact in the health of many women, and in number of cases, in absence of due attention to treatment of the disease, the fibrous nodes in breast can become malignant (Fig. 1).
Normal breast tissues![]()
Hyperplasia (increase in cell numbers)![]()
Atypical hyperplasia
(abnormal increase in cell number, breast tumor markers)![]()
Carcinoma in situ (the cancer cells do not spread
out of the ducts, where they were originated)![]()
Invasive cancers (cancer cells spread out of the
ducts or lobules, from their originated places)
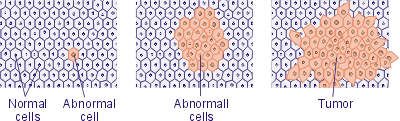
Fig. 1. Different stages of the pathological changes in breast tissues
The importance of atypical hyperplasia as a biological marker for increased risk of developing invasive breast cancer has been confirmed in a multicenter study involving more than 280,000 women [3].
Although mastopathy (mastos = breast, pathy = disease/disorder) is a collective term for all the pathological changes in breast tissues- here under the mastopathy, we'll consider mainly the dishormonal hyperplasias of mammas (FIBROCYSTIC CHANGES), as it is one of the most common benign conditions that affect more than 50 percent of women having palpably irregular breasts, cyclic pain, and tenderness.
Fibrocystic changes occur in the following three major breast tissue elements:
1. Ducts: ductal hyperplasia and cyst formation;
2. Lobules: adenosis (lobular hyperplasia) and sclerosing adenosis;
3. Stroma: fibrosis.
On the basis of these structural changes the dishormonal hyperplasias of breast are classified as follow [3, 4]:
1. The nodulose form
A) Adenofibromas.
B) Fibroadenomas.
2. The diffuse form.
A) Adenosis.
B) Adenosing fibrosis.
C) Fibrosing adenosis:
1) lobular,
2) ductal,
3) fibrotic,
4) cystic,
5) proliferous:
a) adenosis type,
b) papillary type,
c) solid type.
Breast Cancer
Breast cancer develops when cells in the breast begin to grow out of control and can invade nearby tissues or spread throughout the body. Theoretically, any of the types of tissue in the breast can form a cancer, but usually it comes from either the ducts or the glands. Breast cancer is the most common form of cancer in women. The first peak of disease incidence is at 30 to 40 years of reproductive ages. According to statistics, at this period of age the breast cancer cases are 80-100 in every 100,000 women. In older ages, the cancer cases are more frequent- for example, at 50-180 cases and at 65-250 cases in every 100,00 women. According to WHO, every year about 750,000 new cases of breast cancers are diagnosed, and this very disease is the main cause of death of women at the ages of 40-55 [5].
The major risk factors for the women to develop breast cancer are- positive family history of breast cancer in a first degree relatives, history of benign breast disease, age over 40 years, late age birth of first child or nulliparity. The use of long term estrogen replacement therapy or oral contraceptives also slightly increases the risk of breast cancer. Radiation exposure and alcoholic beverage consumption carry high risk of breast cancer. Smoking increase the risk of metastases in lungs [6, 7].
Today, it has been admitted that breast cancer is located 3-5 times more often in women with benign lesion of breast and 30-40 times more often if the women are suffering from nodulose form of mastopathy with signs of epithelial proliferation. About 10-15% of breast cancers are thought to be hereditary [8]. If we look at the genetic levels, the normal cells become cancerous due to certain mutagenic genes. Such changes are found more frequently in the genes BRCA1 and BRCA2 (BReast CAncer Gene 1 and BReast CAncer Gene 2) (Fig. 2).


Fig. 2. Genes, in which mutations take place during breast cancer development
The mutations in BRCA1 take place in about 45% cases of hereditary breast cancers, where as in BRCA2- in about 35% cases. These genes are present in women as well as in men, thus such genetically changes may be passed down from both father and mother sides. Men with BRCA2 genes are also considered to be of high risk to develop breast cancer.
Breast cancer is classified using the TNM system (American Cancer Society)-
Stage 0: is very early breast cancer. The cancer has not spread further ducts [Ductal carcinoma in situ (DCIS)] or further the lobules [Lobular carcinoma in situ (LCIS)].
Stage I: the tumor measures 2 cm or less and has not spread to the lymph nodes, other tissues.
Stage II: the tumor is of sizes 2-5 cm, and/or the cancer has spread to 1-3 auxiliary lymph nodes on the same side; tumors of 5 cm with out any metastases to lymph nodes.
Stage III: tumors less than 5 cm with metastases to 4-9 auxiliary lymph nodes; tumors bigger than 5 cm with metastases to 1-9 auxiliary lymph nodes; tumors that has spread locally (close to the breast) and usually involves the skin, chest wall or 0-9 auxiliary lymph nodes. The metastases to distant organs or lymph nodes are absent in III stage.
Stage IV: the cancer of any size, that has spread to the other parts of the body, most often the bones, lungs, liver, brain or distant lymph nodes.
Inflammatory breast cancer – a form of the ducts carcinoma infiltration. It is so called according to its typical clinical symptoms. The breast appears to be tumorous and skin around it becomes compact, warm, reddening and high tenderness i.e. forms a breast tissue infiltration. It takes place due to fast growth of cancer cells and blockage of lymphatic ducts of breast. In 90% of cases, by the time of diagnosis this type of cancer, the cancer cells already begin to spread through the lymphatic ducts. The prognosis for this type of cancer is most unfavorable, but then it is encountered rarely too.
Breast nodules should be assessed by physical examinations, mammography, aspiration biopsy and pathological tests (estrogen and progesterone receptors, DNA cytometry- flow cytometry or image cytometry, the measurement of S-phase fraction, HER2-neu over expression).
Awareness, regular self examination, early/yearly mammography and GYN checkups are the screening methods to make early diagnosis of mastopathy and breast tumors.
The conventional treatment options available
There is no concise algorithm available for the mastopathy treatments. In every single cases it is highly individualized, however the major approaches are:
a) Hormonal treatments.
b) Non hormonal treatments – drug and surgical treatments.
Hormonal treatments:
As mentioned above, the growth and proliferation of the breast tissues are corresponded with the body levels of the estrogens, progesterone, prolactin, growth hormone, androgen, thyroxin etc. However, the hormonal therapy is directed mainly to correct the estrogenous impact on the breast tissues [10, 11].
Antiestrogens – tamoxifen [6], toremifen, aromatase inhibitors are most common ones which have demonstrated many successful results in treatments of mastopathy as well as in decreasing the risks of cancer developments [11, 13, 14].
Oral contraceptives – the correct choice of the oral contraceptives may not only control the ovulation but also decrease the synthesis of estrogen-receptors in endometrium and balances the drastic hormonal changes during the menstrual cycle. The contraceptive choice should contain the least amount of estrogens and high hystogens. A good regression of mastopathy has been demonstrated by using hystogens in 2 out of 3 cases. The only negative thing is that the course has to be continued for long period- 1 or 2 years before we observe such clinical improvements.
The derivatives of testosterone (linestrinol, norgestrile, dynazol) are more popular than the derivatives of progesterone (medroxyprogesteron acetate – MPA).
Prolactin secretion inhibitors – drugs like bromkriptin, have positive effects in the mastopathy patients with positive TRH-test (thyrotropin hormone test).
LHRH (luteinizing hormone releasinghormone) analogies – their clinical effects are due to their serum estrogen and testosterone lowering effects. Since they have high side effects, they are rather indicated to more severe cases of mastopathy, for example in the severe mastalgia form of mastopathy, when other treatment options bring little improvements.
Note: When mastopathy develops in the patients who are undergoing substitution hormone therapy, additional hormonal treatments may worsen the case.
It is worth mentioning, that proliferatious form of fibroadenoma as well as fibro-cystic or fibromatous mastopathy response little to the applied hormonal therapy, which at the same time possesses many adverse effects.
Non hormonal drug therapies:
In mastopathy besides hormonal disbalances, a considerable impact of limbic system, reticular formations and metabolic processes in the patients also have been noticed.
Thus the complex therapy of vitamin therapy (A, C, E and B); sedatives/anti psychotics; diuretics; peripheral vasodilators; non steroid anti-inflammatory drugs (NSAIDs); correction of nutrition or tonics, and correct choice of brassiere have positive clinical effects in these mastopathy patients.
Unfortunately, these traditional conventional methods of mastopathy treatments do not solve the most important problem- prophylaxis of breast cancer, as very often even a careful observations by doctors can not notice the malignancy of mastopathy on time, where as by half or one year, the process may become irreversible.
Surgical treatments
A needle aspiration – One of the most favorable methods: a small needle is placed into the "cyst" and the fluid is drawn out. This is often for diagnostic purposes; but may serve as therapeutic for larger cysts, if the fluid can be successfully removed.
Usually, the nodules are accurately resected out in mastopathy.
However, in recent times the surgical methods of treatments in mastopathy are becoming less popular, as they do not eliminate the main causes of the disease.
Breast Cancer Treatment Options
The conventional treatment choices available for breast cancers are surgery, chemotherapy, radiation therapy and hormonal therapy [11, 13, 14, 15, 16].
The surgical treatments, depending on the stages, range from Breast Conserving Therapy (BCT); removal of a larger part (but not the whole breast)- segmental or partial mastectomy for early stages (0, I&II) to modified radical mastectomy for more advanced cancers.
Chemotherapy and radiation therapy are usually combined to these surgical treatments. BCT and partial mastectomy always need to be combined with radiation therapy.
Tamoxifen is being used most commonly as hormone therapy in patients with positive estrogen tests, while novel drugs – aromatase inhibitors are the hormonal therapy choice for older aged patients in menopause.
The types, doses of chemotherapy, radiotherapy, hormone therapy and their combinations with surgical treatments depend on the different stages of the cancers
The need of novel approaches for the treatment of masthopathy and breast cancer
The standard traditional methods mentioned above not only do not solve the present world breast problems but also possess many side effects. Surgical method, which suppose to be best solution for coordinal treatments have besides the usual surgical risks and cosmetic defects, can not cure the disease completely- high percentage of relapse cases are not uncommon. Although they might show some control over the initial state of cancers, they practically can not solve the problems of metastases and relapses.
The hormonal treatment option is also not a solution for breast problems; and has many adverse effects. The most widely used tamoxifen has demonstrated some reduction in relapses risks and shrinkage but its use is also complicated by an increased incidence of endometrial hyperplasia/carcinoma, venous thromboembolism, cataracts, and in some cases, emergence of tamoxifen-dependent clones of breast cancer. This has led to the concept of "ideal" selective estrogen receptor modulators (SERMs), drugs that would have the desired, tissue selective, estrogen-agonist or -antagonist effects such as Raloxifene. The efficacy of Raloxifene in the treatments of breast cancer is still under investigations.
One of the novel breast cancer drug options with promising results is so called Herceptin. Herceptin is unique in that, it is directed against a selected HER2 receptors, which are often present on the breast cancer cell surfaces [17]. Herceptin is monoclonal antibody which combine with these cancer cell receptors and block their access to endogen estrogens. As the HER2-receptors located on the cancer cell surface are blocked, the cancer cells stop to grow further, and thus in many cases we observe tumor shrinkage. Besides, herceptin has one more antitumor effects- it is a strong mediator of antibody-dependant cytotoxicity.
The negative points of herceptin are- it covers only 25% of breast cancer cases (HER2 protein over expression rate), and has many side effects including cardiotoxicity- ventricular dysfunction and congestive heart failure.
The other novel drug is XELODA (capecitabine). Xeloda it self can not destroy tumor cells; in the beginning it has to undergo three steps of metabolic changes in the organism (18). The third step–formation of an end product, which possesses cytotoxic effect, takes place inside cancer cells. In this way, the cancer cells becomes a 'factory' which produce toxins against themselves. This not only increases the drug efficacy but also minimizes the possible adverse effects in the organism. These unique qualities of this drug make it so called 'a tumor activated' drug. Xeloda is the first and the only drug of this progressive class so far has been investigated.
By now, cancer scientists have been more and more convinced that immunological correction is the key to defeat cancers [19, 20, 21, 22, 23, 24].
Mammaglobin-A is highly over expressed in breast cancer cell lines. This pattern of expression is restricted to mammary epithelium and metastatic breast tumors. Thus, mammaglobin-A-specific T cell immune responses may provide an important approach for the design of breast cancer specific immunotherapy for the treatment and prevention of breast cancer [25, 26].
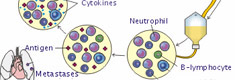
Immune surveillance can be implemented through interaction of immune components with MHC II antigen and costimulatory molecule expression on the surface of breast cancer [27].
Using a new flow cytometric method, an influence of antitumor vaccination on antigen specific T cell immunity in breast cancer patients was studied [28]. The out come results clearly indicate the importance of anticancer vaccinations.
So, following these clinically based facts, it is quite clear that the solution to the current growing problems of breast diseases, particularly mastopathy and breast cancers, is to develop a strong and antitumor specific drug option which:
1. prevents the diseases as vaccine;
2. can solve the problems of metastases, relapses with out having major side effects.
Cancer vaccines are one of the novel R&D approaches which carry these properties and have demonstrated many promising results towards prophylaxis as well as treatments of breast cancers [29, 30, 31, 32].
The antitumor vaccine RESAN is one of such novel drug
How does vaccine RESAN help in breast problems?
The vaccine RESAN, in its basic composition, include glycoproteins which are analogous to fragments of tumor-associated antigens of breast cancer:
1. Ovarian carcinoma antigen CA125 (1A1-3B) (KIAA0049);
2. MUCIN 1 (TUMOR-ASSOCIATED MUCIN);
3. BREAST CARCINOMA-ASSOCIATED ANTIGEN DF3;
4. Cancer associated surface antigen;
5. Adenocarcinoma antigen ART 1;
6. Serologically defined breast cancer antigen NY-BR-15;
7. Serologically defined breast cancer antigen NY-BR-16;
8. CA 19-9.
These glycoproteins included in the composition of the vaccine imitate 6-50 different peptide fragments (each with 7-30 amino acids) of each breast cancer antigens listed above.
The researchers pointed out that the tumor markers such as CEA, CA 125, CA 19-9 and TUMOR-ASSOCIATED MUCIN can be used in monitoring the treatments of breast cancer.
Thus, RESAN – a cancer vaccine due to its unusual xenogenic properties can trigger sufficient (unlike the self weak immunogenic tumor antigens) antitumor specific immune responses showing an effective agent for the prophylaxis and treatment of mastopathy and breast cancers.
Conclusion
Despite of immense on-going research works, the overall growth of breast cancers in the world is still out of control. Around this problem, dishormonal hyperplasias of mammas is one of the most common diseases, which may result into breast cancer.
There are no drugs so far available for conventional use for the prophylaxis of these diseases. The conventional treatment options that are available today in the "breast problem world" have shown only limited successes.
In such conditions, the vaccine RESAN with its' vast antitumor immunotherapeutic activities certainly show the following prevailing advantages over the present conventional methods of mastopathy and breast cancer treatment options:
1. RESAN can be used for prevention of these diseases, especially in those patients, whose relatives had suffered from breast cancers; or to prevent malignancy (degeneration into malignant tumor) and to avoid operations in a number of cases with benign tumors or mastopathy.
2. It can be administered in combination with surgical methods (see the most rational use of Resan) to destroy small metastases and to prevent relapses after the surgical treatment of breast cancers which may result into absolute cures.
3. It can be combined with hormonal therapy or biotherapy like herseptin to increase the overall therapeutic effects and minimize their side effects by reducing the applied doses of the hormone.
4. RESAN can release considerably pain symptoms in most of the cases, there after improves the general health conditions and daily life-style, especially of those patients with highly aggressive or advanced cancers.
5. It does not possess serious side effects and is easy to administer.
Due to this, vaccine RESAN can substantially change the present wretched impression about the cancer treatments associated with hair loss, weakening of hematopoietic system and other unpleasant harsh side effects.
References:
1. Grio R., Cellura A., Germao R. et al. // Minerva Girncolegica. 1998. Vol. 50 N3. P. 101-103.
2. Kotller M. L., Stwrzec A., Carre M. C. et al. // Int J Cancer. 1997. Vol. 71. N 4. P. 595-599.
3. Online management of breast diseases, Benign Breast Lesions. TransMed Network
4. Diagnosis of breast diseases. V.N. Serov, T.T. Tagieva, V.N. Prilevskaya. The Scientific organization of obstetrics, gynecology and perinatalogy (Dir- acad. PAMN V.I. Kulakov). On-line.
5. Siderenko L.N. Mammary Galnd. How to self prevent from breast cancer. 1998.
6. Armstrong, K., Eisen, A., & Weber, B. (2000) Primary Care: Assessing the Risk of Breast Cancer. The New England Journal of Medicine, 342(8), 564-571. Goldhirsch, A., Glick, J.H., Gelber, R.D., Coates, A.S., Senn, H-J. (2001)
7. Breast Cancer Risk Factors. On-line.
8.Weiss MC, Fowble BL, Solin LJ, et al.: Outcome of conservative therapy for invasive breast cancer by histologic subtype. Int J Radiat Oncol Biol Phys 23 (5): 941-7, 1992. [PUBMED Abstract]
9.Wazer DE, Schmidt-Ullrich RK, Schmid CH, et al.: The value of breast lumpectomy margin assessment as a predictor of residual tumor burden. Int J Radiat Oncol Biol Phys 38 (2): 291-9, 1997. [PUBMED Abstract]
10. The drug therapy of fibro-cystic breast disease (mastopathy).
D. Baltinya, A. Srebnii. The Latvian Scientific-Research Institute of Clinical and Experimental Medicine, Riga (Latvia).
11. Keshelava V.V. // New treatment and diagnostic means in oncology. International Medical Journal 5' 2000 p. 457-459.
12. The use of Tamoxifen (Breast Cancer Drug) is Questioned in Dutch Study because of a higher incidence of Endometrial Cancer. By Denise Grady íå íàõîäèò ñòðàíèöó
13. Selective estrogen receptor modulation: the search for an ideal hormonal therapy for breast cancer.
Dhingra K.Hoffmann-La Roche, Inc., Nutley, New Jersey 07110, USA. Cancer Invest 2001;19(6):649-59
14. Oncolinks. Breast Cancer: The Basics. Christopher Dolinsky, MSIV The University of Pennsylvania Cancer Center, May 29, 2002.
15. Meeting Highlights: International Consensus Panel on the Treatment of Primary Breast Cancer. Journal of Clinical Oncology, 19(18), 3817-3827.
16. Hortobagyi, G.N., (1998) Drug Therapy: Treatment of Breast Cancer. The New England Journal of Medicine, 339(14), 974-984.
17. Herceptin® (Trastuzumab). Genentech, Inc.1 DNA Way South San Francisco, CA 94080-4990
18. XELODA® (capecitabine). Mechanism of Action.
19. Kruger, W. et al. Reverse transcriptase/polymerase chain reaction detection of cytokeratin-19 mRNA in bone marrow and blood of breast cancer patients. / J. Cancer Res. Clin. Oncol. 1996, 122 (11), 679-686. 16. Ethier, S. P. et al.
20. Differential isolation of normal luminal mammary epithelial cells and breast cancer cells from primary and metastatic sites using selective media. / Cancer Res. 1993, 53 (3), 627-635.
21. Immunogenicity and immune response in breast cancer. Carasevici E. Department of Immunology, Faculty of Medicine, Gr. T. Popa University of Medicine and Pharmacy, Iasi, Romania. Roum Arch Microbiol Immunol 2001 Oct-Dec;60(4):285-96
22. Natural anti-Gal antibody as a universal augmenter of autologous tumor vaccine immunogenicity. Immunology Today. vol.18, No. 6, june 1997, pp. 281-285. Uri Galili and Denise C. LaTempl.
23. Tumor antigens recognized by T cells. Immunology Today. Thierry Boon, Pierre G. Coulie and Benoit Van den eyde. vol.18, No. 6, june 1997, pp. 267-268.
24. Analysis of endogenous peptides bound by soluble MHC class I molecules: a novel approach for identifying tumor-specific antigens. Eur J Immunol 2002 Jan;32(1):213-22. Barnea E, Beer I, Patoka R, Ziv T, Kessler O, Tzehoval E, Eisenbach L, Zavazava N, Admon A.The Smoler Protein Center, Department of Biology, Technion, Haifa, Israel.
25. Stress, coping, and immune function in breast cancer. Luecken LJ, Compas BE. Department of Psychology, Arizona State University, Tempe 85287, USA. Ann Behav Med 2002 Fall; 24(4):336-44.
26. Identification of HLA-A3-restricted CD8+ T cell epitopes derived from mammaglobin-A, a tumor-associated antigen of human breast cancer.
Jaramillo A, Majumder K, Manna PP, Fleming TP, Doherty G, Dipersio JF, Mohanakumar T. Department of Surgery, Washington University School of Medicine, St. Louis, MO, USA. Int J Cancer 2002 Dec 10;102(5):499-506.
27. Major histocompatibility complex class II antigen and costimulatory molecule expression on the surface of breast cancer cells. Fan P, Wang S, Liu X, Zhen L, Wu Z. Department of General Surgery, First Hospital, Nanjing Medical University, Nanjing 210029, China. Zhonghua Zhong Liu Za Zhi 2002 Jul;24(4):327-30.
28. Impact of high-dose chemotherapy on antigen-specific T cell immunity in breast cancer patients. Application of new flow cytometric method.
Svane IM, Nikolajsen K, Hansen SW, Kamby C, Nielsen DL, Johnsen HE. Department of Oncology, Herlev Hospital/University of Copenhagen, DK-2730 Herlev, Denmark. Bone Marrow Transplant 2002 Apr;29(8):659-66.
29. Pantel, K. et al. / Establishment of micrometastatic carcinoma cell lines: a novel source of tumor cell vaccines. / J. Natl. Cancer Inst. 1995, 87 (15), 1162-1168. 15.
30. The most important part in defeating cancer is a well working immune system... On-line.
31. The present and future of cancer vaccines – a measured perspective... On-line.
32. How to find current cancer vaccine clinical trials?
33. Vecchione A. New and old in prognosis determination. 1993, Nov-Dec, 7 (6B0. p.623-636).
34. Yasasever V., Karaloglu D., Erturk N. Diagnostic value of the tumor marcers in breast cancer. Eur. J. Gynaecol Oncol. 1994, 15(1), p. 33-36.