Immunotherapy - a New Approach in the Treatments of Endometriosis
Endometriosis is a condition where the cells that are normally found lining the uterus are also found in other areas of the body: usually within the pelvis. Each month this ectopic tissue, under normal hormonal control, proliferates and is shed in the same cyclic way as the endometrium. The monthly internal bleeding in the pelvis, unlike the period, has no outlet from the body, which leads to inflammation, pain and the formation of scar tissue.

Fig. 1. The possible localizations of endometriomas
The term endometriosis is derived from the ancient Greek: end- inside, metra- womb and osis- disease. According to different sources, from 6 to 44% of the women in reproductive ages suffer from endometriosis [1]. More over, endometriosis is observed in 30-40% of cases among the infertile women [2, 3]. The "nodules" or "tumors" of endometrial tissue (also called as endometriomas) are found mainly in peritoneum linings of pelvis and ovaries, which appear either in the form of small superficial 'islands' or in the form of epithelial "chocolate" cysts. The growth and progression of endometriomas, just like endometrium, respond to the cyclic changes of estrogen levels. The basic clinical symptoms of endometriosis are dysmenorrhea, dyspareunia (pains during sexual intercourse), pelvic and abdominal pains in correlation with menstrual cycles, leading to one of the frequent complications – infertility [3, 4]. In the last decade, a considerable rise in malignancy preceded by endometriosis has been observed.
In accordance to the American Society of Reproductive Medicine (formerly the American Fertility Society) endometriosis is classified into the following stages:
The stage of endometriosis is based on the location, mass, depth, and size of the endometrial implants, i.e. the specific criteria include:
- the extent of the spread of endometrial implants;
- the extent of pelvic adhesions;
- the involvement of pelvic structures in the disease;
- the degree of the fallopian tube occlusion.
The stage of the endometriosis does not necessarily reflect the intensity of pain experienced, risk of infertility, or other symptoms present. For example, it is possible for a woman in Stage I to be in tremendous pain, while a woman in Stage IV may be asymptomatic. It has been noticed that women who received treatment during the first two stages of the disease had the greatest chance of regaining their ability to become pregnant following treatment.
Endometriosis only a gynecological manifestation of a systemic pathology – it may emerge like tip of a much larger invisible iceberg, one that represents a whole range of health problems that have underlying hormonal and/or immune disorders. Besides the basic symptoms – dysmenorrhea, dispareunia and infertility which are traditionally associated with endometriosis, women with this disease may develop a whole range of other symptoms – high degrees of allergies, chemical sensitivities, susceptibility to infections, mitral valve prolapse and chronic fatigue syndrome [5]. A recently completed study by the American Endometriosis Association has provided strong evidence that there is an increased risk of breast cancer, ovarian cancer, melanoma and non-Hodgkin's lymphoma in women with endometriosis and in their relatives [6]. Women with endometriosis have a higher incidence of thyroid disease including an under active thyroiditis (Hypothyroidism), an overactive thyroiditis (Hyperthyroidism or Graves' Disease), and Hashimoto's Thyroiditis [5]. In addition, other autoimmune diseases such as Rheumatoid Arthritis, Lupus, Multiple Sclerosis, and Meniere's disease are seen somewhat more frequently in women with endometriosis and in their close families, which once again approves that there is an important immune component in endometriosis developments.
Endometriosis and Immune system
Endometriosis can be considered analogous to tumor diseases with the similar involvement of immune system controlling over them. Usually, the normally functioning immune regulatory system of a women checks the outgrowths of ectopic endometrium in any places of the body other than in the endometrial cavity. It (immune system) can destroy the ectopic endometrial cells just like any other neoplastic cells. Where as in women suffering from endometriosis, we observe a dysfunctioning immune system, which allowed the growths of ectopic endometrium. Such undestroyed ectopic endometrial cells may cause inflammation followed by activation of macrophages [7, 8]. The immune alterations in patients with endometriosis are associated with an exaggerated B-cell response and elevated serum levels of autoantibodies. Women with endometriosis have a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium.
The conventional treatment options in endometriosis
The treatment of endometriosis in each individual cases are based on:
- the anamnesis and presence of concurrent diseases;
- clinical symptoms, their intensities;
- stage of endometriosis;
- reaction to the applied drug therapy and other non medicinal treatment methods;
- patient's concern to pregnancy.
The non invasive (conservative) therapy of endometriosis are basically directed towards:
Application of analgesics, for example NSAIDs (Non Steroid Anti-inflammatory Drugs) and other analgesics.
Hormonal therapy: application of gonadotropin-releasing-hormone (Gn-RH) agonists, which inhibits the secretion of estrogens developing so called "medicine caused menopause"; application of Danazol, a synthetic testosterone derivative; application of contraceptive pills containing both estrogen and progesterone which terminates the ovulation and decreases menorrhagia (excessive blood loss during menses).
Surgical methods of treatments of endometriosis include:
Laparoscopic (endoscopic) resections: a tube with optical apparatus called laporascope is inserted into the peritoneum cavity through a small incision, and ectopic growths (endometriomas) are resected.
Laparotomy – more invasive method in which the ectopic growths (endometrioma) are resected taking care not to harm much the surrounding healthy tissues.
Hysterectomy – resection of uterine body (in some cases together with the uterine appendages). In some cases a combined methods are used, for example, laparascopic surgery or laporotomy are performed together with a hormonal therapy.
The antagonists of Gn-RH are new approaches being used for the treatments of endometriosis.
In fact, the researches claim that this group of drugs, in contrast to the commonly used agonists of Gn-RH, do not cause a broad side effects and at the same time exceed in effectiveness.
The aromatase inhibitors – a novel approach in hormonal therapy of endometriosis, also act by decreasing body's supply of estrogen. They were initially used only in breast cancers. They are usually reserved for patients who have already gone through menopause.
Selective Estrogen Receptor Modulators (SERMs) cause estrogenic impacts in selective tissues wherever it is necessary (particularly in cardiovascular and osteo-muscular systems), but they do not have any negative effects in uterine and breast tissues. As were approved in some latest research works in animal models, the application of SERMs prevents from osteoporosis and estrogen induced proliferation of endometrium. During its use in rhesus monkeys, suffering from endometriosis, a considerable shrinkage in uterine size and the sizes of ectopic endometrioma growths were observed.
Similarly, the possible use of Selective Progesterone Receptor Modulators (SPRMs) are under investigations. These drugs possess highly selective impacts on progesterone receptors, and thus it is expected that their long-term administration could decrease the ectopic growths of endometriomas with out having any negative impacts on bone tissues.
The use of Extracellular Matrix Modulators (EMMs) are also of increasing interest among the researchers. The proliferating endometrium produces some specific enzymes, while isolation and destruction of these ferments by EMMs can result into one of the most perspective method of endometriosis treatments.
Terbutalin is successfully used for preventing premature birth, but its effectiveness in releasing the pain syndrome during endometriosis is also being studied.
RU-486 (myphepriston) checks the ability of endometriosis to proliferate in response to estrogen, which makes it possible to use in hormonal therapy against endometriosis.
Angiogenesis is also very important field of research works. As we know any newly formed tissues (like endometrioma – ectopic growths) need additional blood supply. Without sufficient blood supply, the hormonal impacts on these ectopic growths have little effect. Thus, the growths of these ectopic endometriomas can be limited by checking the blood supply to them. The drugs, which decelerate or arrest angiogenesis may also check fibrotic growths or formation of adhesions
Immunotherapy of Endometriosis
The conventional methods practiced today in the endometriosis treatments are either surgical or hormonal in their nature. But, since there is a considerable role of immune changes in the origin of endometriosis, we can not expect a full recovery from the surgical approach alone. The hormonal treatment is also not a true etiological or pathological approach in the treatments of endometriosis; it ensures only temporary treating effects.
Many research works of recent years have demonstrated a large number of immune abnormalities in the endometriosis patients, and so it can be assured that it is the defect in immune system, which gives rise to this disease. This assumption is further supported by the observation of immune defects already present in the mildest forms of the disease. So, the immune etio-, pathogenesis of this disease suggests the implied successful immunological treatment approach for endometriosis.
The target of immune therapy is to "normalize" the function of activated NK cells, and increase the T-cell responses against the ectopic endometrial growths.
A very new approach – an immunotherapy using tumor vaccine RESAN, which triggers specific T-cell immune response against endometriosis has been elaborated by a clinical research group – The ScientificResearch Enterprise Resan – showing many promising results [23, 24] .
The out growth tendency of endometrioma gives high risks of relapses and spread of the primarily implanted ectopic masses to distant places. This invasive nature of these cells shows some common properties with malignant tumors of ovary and uterine body. It has been elicited that the low histological differentiation, high risk of local invasions and metastases correspond with the high expression of receptor growth factors, tumor associated antigens CA125, CA15-3 and low expression of molecules for cellular adhesion – E-cadherine [28, 29, 30, 31]. Since the ovary tumors, uterine tumors and endometriosis all have defects in proliferation control mechanism by immune system, we recommend an application of tumor antigen imitators (vaccine RESAN) to correct and strengthen the immune system of endometriosis patients.
Next we represent a short reviewed protocol of our clinical assessment of 22 patients. Among them 12 were suffering from genital endometriosis – adenomyosis, posterio-isthmus of uterus form of endometriosis, external genital endometriosis; 10 were suffering from combined forms- endometriosis and uterine myoma; and in 2 cases were found ovarian cysts [22]. All the patients have had different treatment approaches including hormone therapy, course of immunomodulators, antioxidants therapy etc. But, the effects from the applied therapy were not prominent; the remission periods were limited to 2-3 months after the end of such therapy. Moreover, there was basically symptomatic improvement (some release in pain symptom and certain improvements in general condition). Following such short-time and inefficient clinical improvements from the previous therapies, we gave them a course of immunotherapy using xenogenic vaccine RESAN, which imitates the antigens of endometriosis and uterine myomas [24]. The vaccine was administered subcutaneously, in single-shot. The patients were examined before and after the vaccination, pain symptoms were evaluated according to Mac Laverty System:
Pain in pelvic region, not associated with sexual intercourse or menses (3 units – severe, sharp, almost constant in which the patients were compelled to take analgesics; 2 units – moderate, tolerable, appreciable discomforts during the whole period of menses; 1 – mild, occasional discomforts or during pre menses period);
Algodysmenorrhea (3 units – severe, compels the patients to remain in bed for one or more days; 2 units – moderate, compels the patients to remain in bed for several hours in a day, occasionally loose working-capacity, 1 unit – mild, with certain loss of working capacity);
Dyspareunia – Pain during sexual intercourse (3 units – severe pains, have to avoid sexual intercourses; 2 units- moderate, have to interrupt sexual intercourse, 1 unit – tolerable pains);
Dysmenorrhea – (3 units – blood-smears seen 4-7 days pre menses; 2 units – blood-smears seen 1-3 days pre menses; 1 unit – occasional appearance of blood-smears pre menses);
Intensity of menses (3 units – copious menses, 2 units – moderate, 1 unit – scant menses).
Bimanual examinations, ultrasound examinations of pelvis were conducted in all the vaccinated patients. The serum tumor markers (CEA, CA-125, CA19-9, CA-15-3), antigens of hepatitis virus B (HIV-B), antibody titers to Chlamydia trachomatis, Toxoplasma gondii, Mycobacterium tuberculosis and HIV-C were measured through immunoferment analysis method before the vaccination and during the follow-ups in one month later.
Before the treatments, the pain symptom was in average 9 units in all these 22 patients, who were suffering from genital endometriosis and in combined form- endometriosis with uterine myoma. Dysmenorrhea was evaluated as 3 units in all these patients.
Heterogenetic consistencies of endometriosis in different stages, areas of high tenderness on uterine walls and increase in uterine body volume were observed during bimanual examinations and ultrasound examinations of pelvis. In the uterine isthmus, pronounced swellings and increased pains were noted. In 12 patients with endometriosis genitalia were found adenomyosis III-IV stages during ultrasound examinations. In the patients with combined forms (endometriosis and myoma uterine), together with hetreogenic mosaic structures, some interstitial, subserosis and submucosis nodes (in 5 cases) of 22-23 mm diameter were found. The uterine size were enlarged up to 9-12 week sizes in all cases.
The tumor markers were elevated with in benign tumor limits, in all patients before the treatments. The average value of CA-125 in the group as a whole was 44.7±1.34 U/mL, CA-19-9 28.9±1.5 U/mL, CA-15-3 53.2±4.3 U/mL, CEA 1.29±0.38 ng/mL.
After one month, all the patients were followed up. During bimanual examinations of the patients suffering from endomeriosis, the uterus sizes were shrank considerably and endometrium tissues became more homogenous with small areas of tenderness. In the cases of uterine myoma, we observed considerable shrinkage in nodules and uterine body sizes. Where as in cases of endometriosis genitalia, the positive results were even more pronounced. During ultrasound examinations of genitalia, there were observed a marked reduction in genital endometrioma and uterine sizes in patients suffering from endometriosis, where as in patients suffering from endometriosis in combination with uterine myoma, we noticed a decrease in interstitial, subserosis and submucosis node sizes (down by 16 mm). In all cases, the uterine body sizes were shrunk to 5-11 week sizes (Fig.2).

Fig.2 Sizes of uterus
In 7 patients were observed normalization of uterine body size, while in ultrasound examination there was a complete disappearance of myoma nodules. Similarly, in 2 patients we observed a complete disappearance of ovary cysts of sizes from 14.5 cm 3 to 26.4 cm 3 after the vaccination.

Fig. 3. The intensiveness of the pain-symptomThe pain-symptom in the patients suffering from endometriosis as well as in combined forms (endometriosis and uterine myoma) was relieved from 9 down to 5-7 units (in a 9 units system) (Fig.3).

Fig. 4. Dysmenorrhea
One month after the vaccination, the severity of dysmenorrhea was reduced to 1 unit in the patients with endometriosis as well as in those with combined forms (endometriosis and uterine myoma) (Fig. 4).
We did not observe any clinical declines in direct correspond to vaccination in the patients. Patients rather admitted a decrease in edema, pain releases in the areas of estrogen-dependant organs, betterment in general health conditions and overall lifestyle.
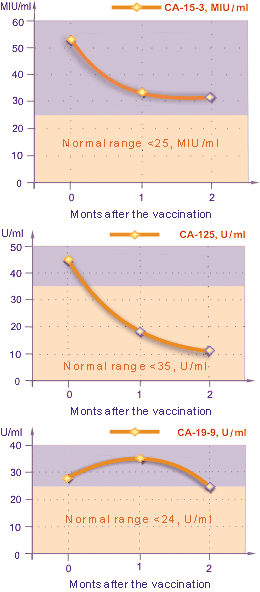 Fig.5 Change in tumor marker levels
Fig.5 Change in tumor marker levels
The changes in the tumor marker levels varied depending on the types of tumor markers (Fig. 5). The CA19-9 level after the vaccination, in average, went up by 23% (P < 0.05) from the initial value during the first month, but after two months it became 15% less than the initial value. The CA-125 level, in average, dropped steadily down to 25% (P < 0.05) from the initial value. The CA15-3 level fell down to 38% (P < 0.05) from the initial value, already in the first month after the vaccination. The average value of CA-15-3 in 2 months after the vaccination was 31,0 U/mL (P < 0,1%). In overall, these changes in tumor marker levels showed positive results of immunotherapy.
After evaluating closely the results of above clinical assessment, we came to conclusion that the application of vaccine RESAN considerably improved the general health condition and overall parameters of objective examinations in all the vaccinated patients who were under our observation.
We recommend the patients to examine for chlamidian infections, tuberculosis as well as for hepatitis B and C carriers before the vaccination. In case of spotting high titers of antibodies against these infections, it is necessary to carry out a "sanitarying treatment" of patients (using antibiotics, interferons, interleukines) before vaccination.
The immunotherapy of endometriosis and its combined form with uterine myoma by xenogenic vaccine RESAN results into regression or complete destruction of endometriomas, myomatosis nodules and cystic formations of ovary.
2. Publication in RF: RESAN in endometriosis treatment
4. RESAN in Przegląd Menopauzalny 2007; 5: 283–288
5. Scientific works of expert in the fiels of endometriosis Szymanowski K.
References:
1. Gleicher, N. (1995). Immune dysfunction - a potential target for treatment in endometriosis. British Journal of Obstetrics and Gynaecology. 102 (Suppl.12), 4-7.
2. The Oxford Clinic – The Endometriosis Treatment Centre. Online.
3. IVF.com, Atlanta, USA. – Endometriosis Association, Education Support Research. Online.
4. Article: Uterine fibroids. Review Date: 04/09/01 Reviewed By: Catherine S. Bradley, M.D., Department of Obstetrics and Gynecology, University of Pennsylvania Medical Center, Philadelphia, PA.
5. Ballweg, ML. (1995). The Endometriosis Sourcebook. The Endometriosis Association. Contemporary Books.
6. Duczman, L. & Ballweg, ML. (1999). Endometriosis and Cancer: What is the connection? Endometriosis Association International Headquarters.
7. Senturk, LM. & Arici, A. (1999). Immunology of endometriosis. Journal of Reproductive Immunology. 43(1) 67-83.
8. Vinatier, D., Cosson, M. & Dufour, P. (2000). Is endometriosis an endometrial disease? European Journal of Obstetrics, Gynaecology and Reproductive Biology. 91(2) 113-25.
9. Paul Dmowski, MD, Director, Institute for the Study & Treatment of Endometriosis (ISTE), Oak Brook, IL.
10."Deep endometriosis conundrum: evidence in favor of a peritoneal origin," Fertil Steril 2000 May;73(5):1043-6 (ISSN: 0015-0282) by Vercellini P; Aimi G; Panazza S; Vicentini S; Pisacreta A; Crosignani PG.
11."Liver Health & Endometriosis," Julia Chang, M. Sc.
12. The Center for Endometriosis Care, Atlanta, GA. Online.
14. "Endometriosis 2000: a Report," by Dr. Mark Perloe. Online.
15. "Non-Invasive Diagnostic Detection of Endometriosis," NIH Grant # 1 RO1 CA96575-01, submitted by Rosalyn Blumenthal, Ph.D., Member/Director Tumor Biology, Garden State Cancer Center.
16. Professor Stephen Smith, Head of investigation of cellular, molecular and genetic factors which regulate angiogenesis and embryo implantation, University of Cambridge/Department of Pathology. On-line.
17. Deborah Metzger, Ph.D., Director, and Andrew Cook, M.D., Co-Director, Helena Women's Health Center. On-line.
18. "Immunopathology of Endometriosis," by The Reproductive Research Center at the Cleveland Clinic Foundation, Cleveland, OH. On-line.
19. Gleicher N. Immune dysfunction – a potential target for treatment in endometriosis. Br J Obstet Gynaecol 1995 Oct;102 Suppl:12:4-7.
20. Gleicher N, Pratt D. Abnormal (auto)immunity and endometriosis.
Int J Gynaecol Obstet 1993; 40 Suppl: S21-7.
21. Gleicher N. Endometriosis: a new approach is needed. Center for Human Reproduction, Chicago, IL. Hum Reprod 1992 Jul; 7(6):821-4
22. The vaccinotherapy of patients with mastopathy, endometriosis and mioma of uterus A. Ovsienko, H. Doronina , V. Yanchenko. Journal of Immunopathology: http://iaci.ru/journal/ http://iaci.ru/journal/issue2/full_text/sec6ar2.pdf
23. Novikov D.K., Novikiva V. I., Derkatch U.N., Novikov P.D. The basic immune corrections. Vitebsk, 1998, pg. 106.
24. Yantchenko V.V., Yantchenko A.V., Yantchenko L. K. Immitators of the tumor antigens. The patent committee RB, appl. N970547, 1997.
25. Immunology, Infertility and IVF, Sher Institute for Reproductive Medicine. On-line.
26. Endometriosis Protocol. On-line.
27. Endometriosis: clinical symptoms, diagnosis and treatments. R.A. Saidov. // Department of obstetrics and gynaecology MMA of I.M. Sachenov. On-line.
28. Gaetje R. Kuteian S., Herrmann G. Invasiveness of endometriotic cells in vivo. // Lancet, 1995, V346, P463-1464.
29. Gamallo C., Palacios J., Suarez A. Correlation of E-cadherin expression with differentiation grade and histological type in breast carcinoma. // Am. J. Pathol., 1993, V 42, P987-993.
30. Inoue M., Ogawa F., Miyata M. Expression of E-cadherin in normal, benign, and malignant tissues of female genital organs. // Am. J. Clin. Pathol., 1992, V98, P76-80.
31. Jimbo H., Hitomi Y., Yoshikawa F. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cyst. // Am. J. Pathol., 1997, V 50, P73-1178.
32. Sayunov M.A.Clinical symptoms, diagnosis and treatments of nodulous type of adenomyosis. // Obstetrics and gynaecology- 1987. N_3. – pages 34-36.
33. Lapauxov D.A. Clinical and diagnostic moments in combined forms of benign tumors of uterus in pre menopause periods: Auto abstracts for PhD candidates, 14.00.01. – M., 1992. pg. 33..
34. New findings on pathogenesis of internal endometriosis. // M.M. Daminrov, B.I. Bakuleva and others. // Obstetrics and gynaecology-1993. ¹ 5. – pages 28-32.
35. Ferenczy A. Pathophysiology of adenomyosis. // Hum. Reprod. Update. - 1998. - Vol. 4, ¹ 4. – pages 312-322.